News Story
Aerosol Containment Chamber to Make COVID-19 Intubations Safer
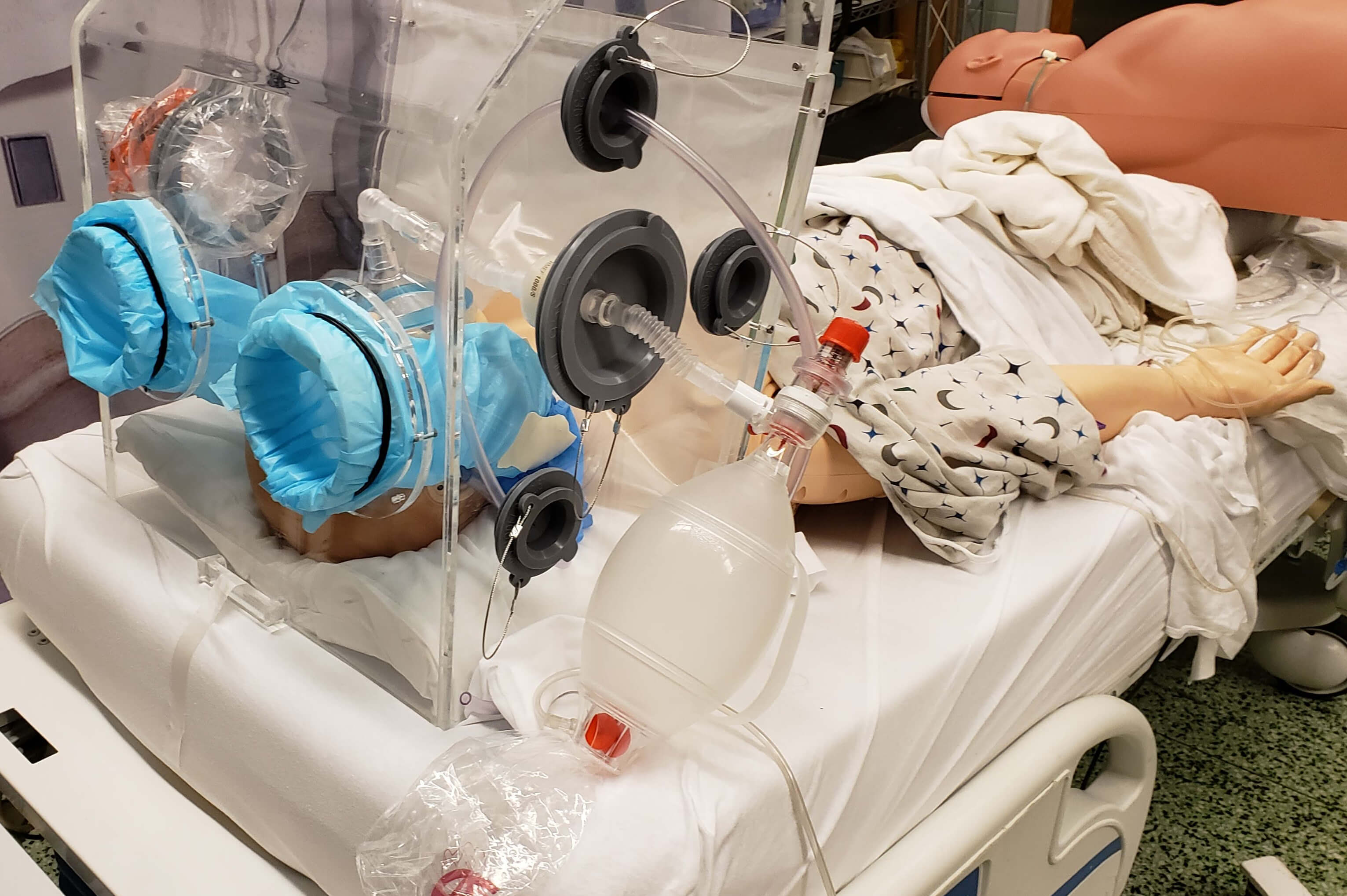 A team of University of Maryland researchers – including School of Medicine faculty and a mechanical engineering alum – have developed a novel version of what is commonly referred to as an intubation box, or an aerosol box. Their patent-pending device is designed to serve as an added protective barrier between a COVID-19 patient and medical staff, particularly when a patient undergoes an intubation procedure to be placed on a ventilator.
A team of University of Maryland researchers – including School of Medicine faculty and a mechanical engineering alum – have developed a novel version of what is commonly referred to as an intubation box, or an aerosol box. Their patent-pending device is designed to serve as an added protective barrier between a COVID-19 patient and medical staff, particularly when a patient undergoes an intubation procedure to be placed on a ventilator.
The intubation procedure itself carries a high risk of virus spread to healthcare workers. This is because a cough or sneeze while placing the breathing tube can spray droplets and aerosols up to 25 feet in the matter of a second, potentially infecting clinicians with the virus.
To safeguard against potential exposure to the virus, standard intubation boxes are made of clear plastic and designed with two holes on the back side to allow the clinician to insert his or her hands into the box to perform the intubation procedure. While these intubation boxes serve as respiratory droplet barriers to the person performing the airway intubation procedure, they do not prevent the spread of aerosols and micro-droplets from dispersing into the room. This is partly due to the fact that particles can still escape from the open front side of the box and two access holes, and remain hanging in the air, particularly if the intubation procedure is performed in a room without adequate ventilatory circulation. Even more, the aerosols generated under the box may remain there until the box is removed – at which point, there is a high likelihood that many particles will waft out into the room.
To address this risk, University of Maryland School of Medicine Assistant Professor of Anesthesiology Jason Brookman, M.D., University of Maryland Medical Center Nurse Anesthetist Bonjo Batoon, CRNA, Pathotrak Head of Engineering Ethan Reggia (‘16, mechanical engineering), Department of Mechanical Engineering Assistant Professor Axel Krieger (Robert E. Fischell Institute for Biomedical Devices/Maryland Robotics Center), and Fischell Institute Senior Research Engineer Kevin Aroom have teamed up with Terrapin Works. Together, the group designed a novel improvement to the standard aerosol box by creating a semi-sealed containment chamber that would be placed over the patient’s head during intubation in a similar fashion. The chamber is connected to the hospital vacuum system via tubing, which creates what’s known in the medical world as a scavenger system. Put simply, this suction system works in a similar fashion to the fan in a home bathroom that removes steam from the bathroom during a hot shower.
Even by conservative estimates, the group’s suction system can can exchange the three to four cubic feet of air volume contained inside the chamber within five minutes. This allows the clinicians or medical staff to safely remove the chamber shortly after the intubation or extubation procedure is performed, thereby minimizing exposure to expelled virus particles in the room.
The research team also made critical adjustments to the aerosol box by using disposable arm-length gloves to virtually eliminate the risk that aerosol particles can escape via the access holes. The group’s design also incorporates additional access ports to allow other medical equipment to be inserted, as needed, through separate tight-sealing diaphragms.
While the group’s negative pressure intubation shield is a bit more robust than the standard aerosol box, it is intended to be manufactured using acrylic and silicone, and a basic suction system - which makes it low-cost and easy to manufacture.
Brookman, Reggia, and Krieger have collaborated with nearly 100 medical professionals – including clinicians at the University of Maryland Medical Center, Johns Hopkins University, Walter Reed National Military Medical Center, and Children’s National Hospital – to fine-tune their design to meet clinical needs for safety and ease of use. The group submitted an application to the U.S. Food and Drug Administration (FDA) for an Emergency Use Authorization to allow hospitals to soon use the device.
In addition to Brookman, Reggia, and Krieger, Terrapin Works staff – including both graduate and undergraduate researchers who are working remotely from their homes – have made tremendous contributions to this work.
Reggia, a mechanical engineering alum, continues to serve as a principal engineering consultant to Terrapin Works.
Additional project updates will be shared on the A. James Clark School of Engineering COVID-19 response website as they are made available.
Published May 28, 2020